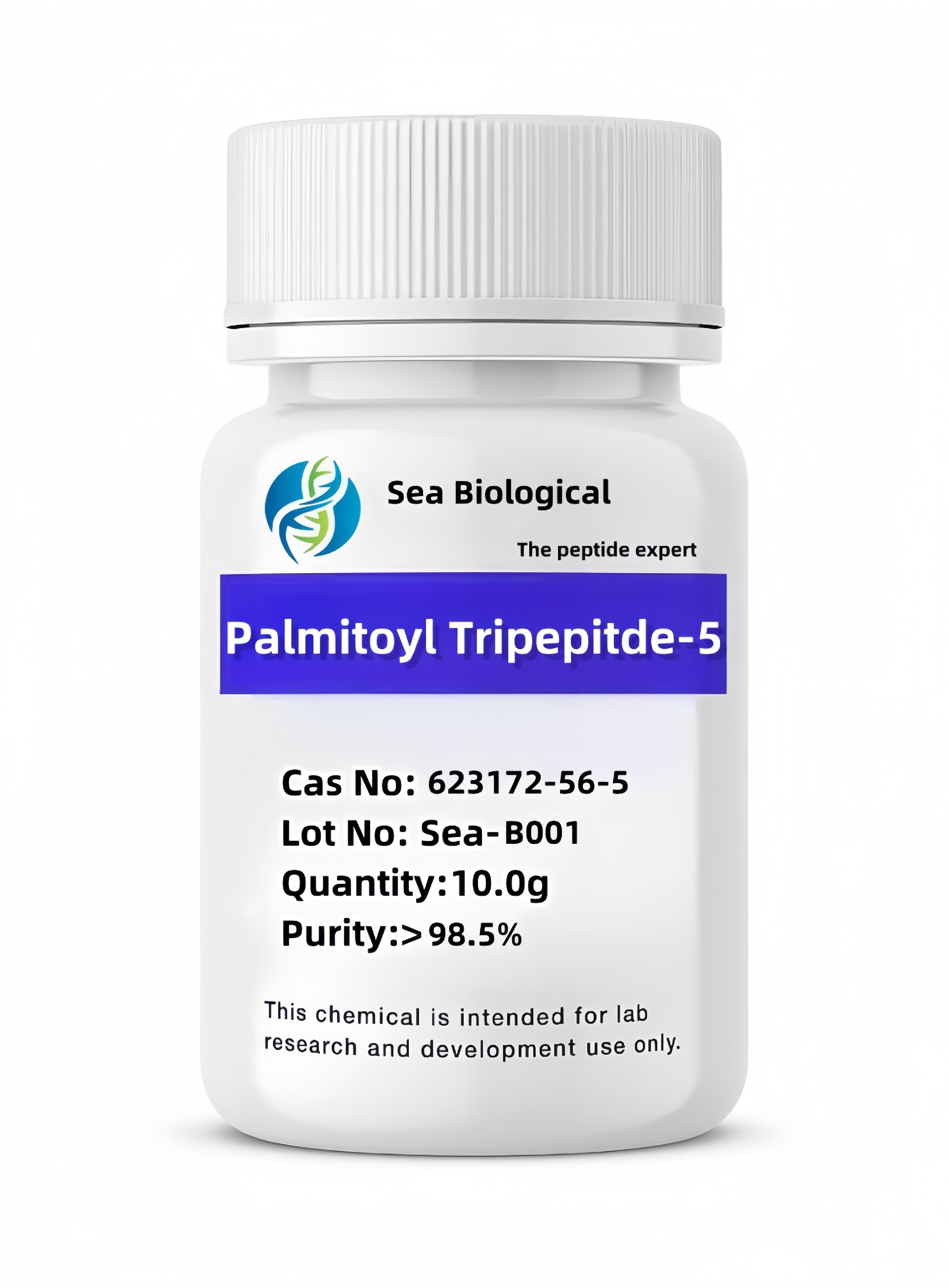
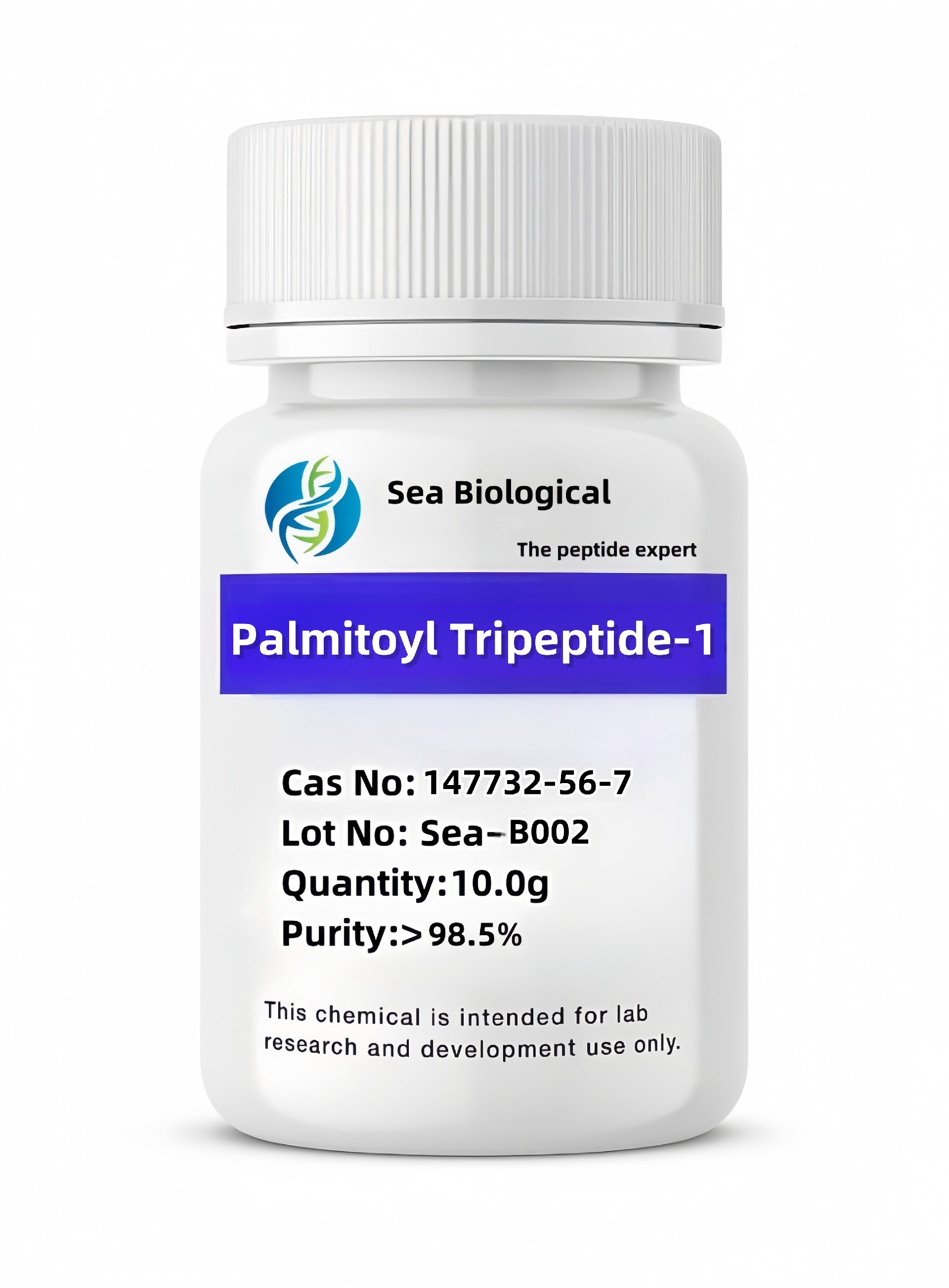
Ganirelix
CAS:
129311-55-3
Purity:
99.0%
Molecular Formula:
C84H121ClN18O17
Molecular Weight:
1570.319
Boiling Point:
N/A
Density:
N/A
Vapor Pressure:
N/A
LogP:
Melting point:
Flash point:
Keywords:
Keywords:
Peptide | Dietary supplement | Pharmaceutical Intermediate
Category:
Ganirelix
Ganirelix acetate was introduced in Germany as prefilled syringes for subcutaneous injections that inhibit premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation. This decapeptide analog of luteinizing hormonereleasing hormone (LH-RH) is the second third-generation LH-RH antagonist to be launched after citrorelix (Asta Medica). This highly bioavailable compound immediately blocks the endogenous release by the pituitary gland of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), the hormone that induces ovulation. After discontinuation of the treatment, the pituitary-gonadal function is rapidly recovered due to its short-half life. As a consequence, ganirelix at daily doses of 0.25 mg S.C. efficiently prevented LH surges during clinical trials in infertile women under controlled ovarian hyperstimulation with recombinant FSH before in vitro fertilization or similar reproductive techniques. Unlike first and second-generation gonadotropin-releasing hormone antagonists, ganirelix has minimal histamine-releasing effects thus avoiding the formation of edema of the face and extremities.
previous page
next page
previous page
next page
Recommended Products